Phone Number
(03) 9466 2803
Email Address
sales@surgisupplies.com.au
(03) 9466 2803
sales@surgisupplies.com.au
Surgi Supplies is an importer of a range of high quality branded sterile and unsterile medical devices and hospital consumables which are supplied to distributors throughout Australia.
We import all the brands your clients know and trust: Medicoplast, Epiglu, Warwick Sasco, and Spirit.
We aim to meet or exceed our clients’ expectations with regards to excellence in quality and timelines.
We supply a wide-range of high quality, cost-effective branded medical devices to distributors throughout Australia.
View Our Product Ranges
For further information on our product ranges, please click ‘More Info’ to view our supplier catalogues, or ‘View Category’ to view our respective product category pages.
A wide range of Reusable Autoclavable Bowls, Instrument Trays, Kidney Dishes, Bedpans, Urinal bottles and more. Polypropylene products are widely used in CSSD, Operating Rooms and Surgery. Great quality products manufactured by CE certified Warwick Sasco. Warwick is available for distribution and wholesale supply throughout Australia.
Click “More Info” for product information on Warwick Sasco.

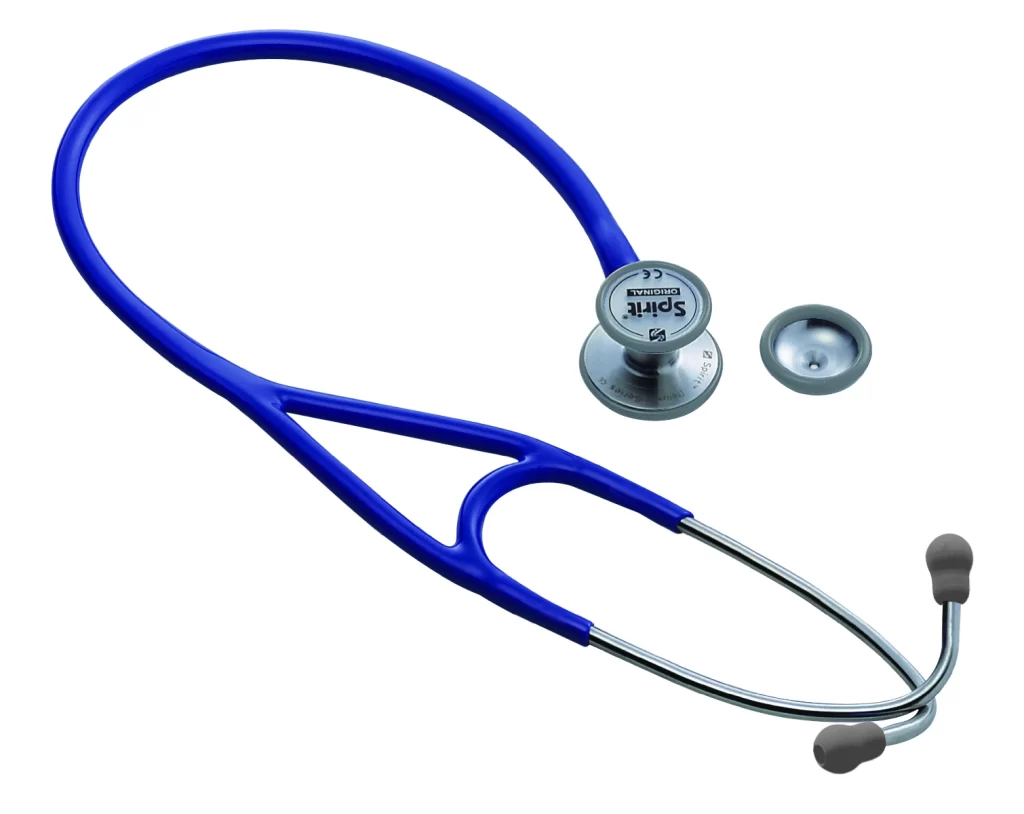
A wide range of Spirit Stethoscopes, Sphygmomanometers and related spare parts including cuffs, bulbs, connectors, bladders for Sphygmomanometers. Manufactured by CE certified Spirit and TGA registered, quality is assured. SPIRIT is available for distribution and wholesale supply throughout Australia.
Click “More Info” for more information on Spirit.
EPIGLU skin adhesive is used instead of sutures in wound treatment. Manufactured by CE certified Meyer Haake and is TGA Registered. EPIGLU is available for distribution and wholesale supply throughout Australia.
Click “More Info” for product information on Epiglu.

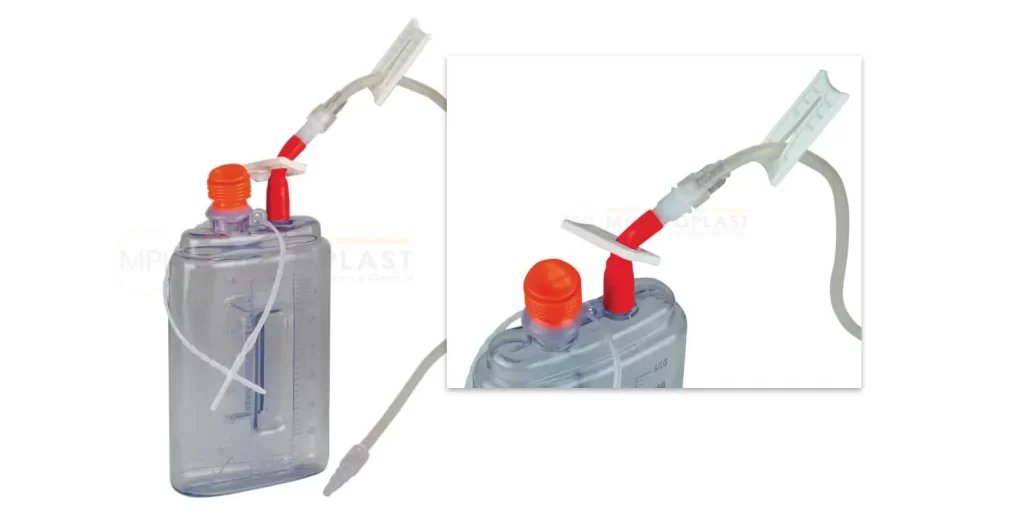
A range of wound drainage and components. Such as drain tubing, trocars, connectors, and suction bottles used in general surgery. Manufactured by CE certified MEDICOPLAST International GmbH and is TGA registered. MEDICOPLAST is available for distribution and wholesale supply throughout Australia.
Click “More Info” for product information from Medicoplast.
HEINE designs and manufactures a full range of high quality primary examination instruments for various medical specialities including Laryngoscopes, Otoscopes, Ophthalmoscopes and more.
Click “More Info” for product range from Heine.

A wide range of high quality German bandages for various medical purposes are available. A range of synthetic cast will soon be available from Surgi Supplies.
Click “More Info” for product range from Q Bandage

We specialise in supplying diverse health industries with high quality products. Our expertise spans various sectors, ensuring tailored solutions.
Below are just a few of the brands we import






Surgi Supplies is a leading manufacturer and importer of premium medical devices and hospital consumables, serving distributors and healthcare providers across Australia.
Our extensive range includes both sterile and unsterile products, featuring renowned brands known for their quality and reliability. Specialising in cost-effective solutions, Surgi Supplies offers a diverse selection of medical essentials, including medical holloware, wound drainage, synthetic casts, skin tissue adhesives, bandages, stethoscopes & sphygomanometers and more.
Our mission is to provide the Australian healthcare industry with access to cost-effective solutions, ensuring the highest standards of care and patient well-being. All our medical products comply with the regulations of Therapeutic Goods Administration (TGA), and other regulatory bodies where applicable.
Vision and Goals: Surgi Supplies Pty Ltd is committed to manufacturing and importing of safe, effective and high quality medical devices. The goal of the company is to maintain a passion for continuous improvement and continuously improve processes and training related services.
Mission: Surgi Supplies endeavour to consistently meet or exceed our clients’ expectations with regards to excellence in quality and timelines, by continuously improving and updating the skills and resources needed for demand, Training and Development.
Commitment: Surgi Supplies commitment to quality is entrenched in the basic philosophy of the company. This commitment encompasses the entire operation; it is not restricted to the product quality but extends to the whole company performance. The company is committed to comply with the regulations of Therapeutic Goods Administration (TGA), and other regulatory bodies.
Our clients feedback reflects our steadfast commitment to providing excellence in quality and service.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua proin aliquet maurisa eiusmod tempor incididunt.

Designer
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua proin aliquet maurisa eiusmod tempor incididunt.

Housewife
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua proin aliquet maurisa eiusmod tempor incididunt.

Programmer
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua proin aliquet maurisa eiusmod tempor incididunt.
Please fill out the adjacent contact form if you have any enquiries, or are interested in becoming a distributor.

Surgi Supplies proudly supplies health care providers across the country with high-quality, reliable and affordable medical supplies through our distributor network.



Sed ut perspiciatis unde omnis iste natus feria delavirot voluptatem accusantium.
Copyright © 2023 SURGI SUPPLIES PTY LTD. All rights reserved.
ABN: 20 669 389 655
Website by Screwloose Digital
Audrey StevensonTailor